Volume 37 Number 3 | June 2023
Case Study
Jasmine Boykin, MSPH, MLS(ASCP)CM
 Case History
Case History
A 69-year-old Caucasian American male with a history of atherosclerotic heart disease, hypercholesteremia, gastro-esophageal reflux disease (GERD), and prostatic cancer presented to the emergency department with a sore throat, cough, nasal congestion, headache, chills, and a near syncopal episode. The patient’s chief complaint was weakness and fatigue. He noted that the symptoms began four days prior to his ER visit and he tested positive for COVID-19 two days prior.
Review of systems was unremarkable. Initial vital signs revealed a slight elevation in blood pressure. The physical examination and chest X-ray were unremarkable. Urinalysis studies were also unremarkable. Venipuncture blood collections were ordered and drawn for complete blood cell count (CBC) with differential and a basic metabolic panel (BMP) performed via point of care (POC). Initial CBC studies were unattainable due to cold agglutinin like presentation within the sample. The sample was prewarmed at 37.0°C for one hour and a warm diluent replacement technique was performed. Upon centrifugation of the specimen, hemolysis was noted, and the specimen was rejected and re-collected.
Initial POC-BMP studies were remarkable for hemoglobin concentration and potassium levels. The patient’s initial hemoglobin reading from the i-STAT POC instrument was a critical value of 4.6 g/dL. An order for packed red blood cells (pRBC) was placed immediately to the blood bank department. The patient’s potassium level was a critical value of 6.7 mmol/L. However, these results were falsely decreased and falsely increased, respectively, due to the presence of hemolysis. Therefore, the initial laboratory results had to be rejected and performed again. The succeeding venipuncture collections were collected via blood warmer, which reduced the amount of hemolysis and cold agglutinin like presentation found within the specimen. However, advanced laboratory techniques were still employed to the specimen to obtain accurate results.
Upon employment of these specialized techniques, the CBC studies were obtained and revealed a hemoglobin concentration of 10.5 g/dL; the pRBC order was canceled. The chemistry analysis was also corrected to a potassium level of 5.5 mmol/L. The patient’s vitals remained stable after being treated for dehydration, and he was discharged the same day.
Results
The initial CBC hemogram revealed several R flags in all the red blood cell (RBC) indices, white blood cell (WBC) differential, and platelet parameters were invalid. The technologist checked the sample for clots. The sample was proven to be severely agglutinated under the microscope (Figure 1).
Figure 1: Review of the peripheral blood smear at 50x magnification showing severe agglutination. This figure shows the initial peripheral blood smear obtained by the initial specimen collected without a heel warmer. Image Citation: Taken by Boykin, Jasmine. Date: August 4, 2022. Location: Saint Anthony Hospital
The sample was then placed in the heat block set at 37.0°C and incubated for one hour. The sample was re-run on the hematology analyzer after the one-hour incubation. The hemogram results remained unchanged. The technologist canceled the CBC order and notified the registered nurse that the test was canceled due to the presence of hemolysis and that the specimen had strong agglutination and needed to be re-collected using a heel warmer. The chemistry BMP add-on results coincided with the hematology findings and the sample had to be re-collected (Table 1).
Table 1. Initial BMP of COVID-19 Positive Patient
Assay (units)
Patient Test Results
Reference Ranges
Interpretation
HgB (g/dL)
4.6 g/dL
14.0-18.0 g/dL
Critical Low
HCT (%)
13.0%
40.0-54.0%
Low
K (mmol/L)
6.7 mmol/L
3.5-5.1 mmol/L
Critical High
NA (mmol/L)
136 mmol/L
136-145 mmol/L
Normal
Chloride (mmol/L)
105 mmol/L
98-109 mmol/L
Normal
tCO2
27 mmol/L
21-31 mmol/L
Normal
Ionized CA
1.12 mmol/L
1.10-1.30
Normal
References are from the technical standard operating procedures of BayCare Health System, Department of Laboratory Services4. HgB, hemoglobin; HCT, hematocrit; K, potassium; NA, sodium; tCO2, total Carbon dioxide; CA, calcium; BMP, Basic metabolic panel. This table contains the initial results reported prior to re-collection of specimens.
At the newly collected specimen’s arrival, the hematology technologist visibly checked the sample for clots or agglutination. The specimen contained minimal agglutination. The technologist pre-warmed the specimen and performed a pre-warm diluent replacement prior to running the sample. The advanced laboratory techniques were successful, and results were obtained (Table 2).
Table 2. CBC Hemogram
Indices(units)
Patient Test Results
Reference Ranges
Interpretation
WBC(x 103/uL)
8.2 x 103/uL
4.5-11.0 (x 103/uL)
Normal
RBC(x 106/uL)
3.21 x 106/uL
3.21 x 106/uL
Low
HGB
10.5 (g/dL)
14.0-18.0 g/dL
Low
HCT (%)
30.0(%)
40-54.0%
Low
MCV (fL)
93.4 fL
80.0-100.0 fL
Normal
MCH (pg)
32.7
26.0-32.0 pg
High
MCHC (%)
35.0
31.0-36.0 %
Normal
RDW-CV (%)
14.0
11.5-14.5 %
Normal
PLT(x 103/uL)
317 x 103/uL
140-450 (x 103/uL)
Normal
MPV (fL)
7.1
6.5-13.0 fL
Normal
References are from the technical standard operating procedures BayCare Health System, Department of Laboratory Services21. WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; PLT, platelets. CBC, Complete blood Count. This table contains the results that were reported post recollection of specimens.
Being that the only critical chemistry result was the potassium levels, the physician only re-ordered a potassium test to be performed. The new potassium level was reported to be 5.5 mmol/L.
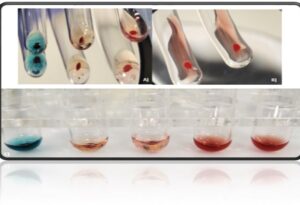
The decision to utilize the heel warmer to re-collect the patient’s sample was successful. It improved the analytical and post-analytical outcomes. As for the immunohematological results, the antibody was reported as negative. The forward and reverse ABO/RH group typing resulted in indeterminate. This indicated an ABO discrepancy. Strong spontaneous agglutination via solid phase occurred, and all cells were positive except for the control (Figure 2).
Figure 2: A) Shows an image of the patient’s forward group typing tube reactions with reagent antisera and patient’s red cell with the tubes. B) Shows an image of the patient’s reverse group typing tube reactions with the reagent red cells and patient plasma. C) Shows the full ABO/Rh group typing reactions. All images show positive for agglutination reaction in each tube demonstrating spontaneous agglutination which indicates an ABO discrepancy has occurred. Image Citation: Taken by Boykin, Jasmine. Date: August 4, 2022. Location: Saint Anthony Hospital.
The technologist performed a pre-warm technique to resolve the ABO discrepancy. This technique resolved only the forward group typing ABO discrepancy. Agglutination was still present in the reverse or back type of the ABO/RH typing. A saline replacement on the reverse type was performed to resolve the discrepancy. The patient ABO discrepancy was resolved, and the patient type matched the patient ABO history on file.
COVID-19 Epidemiology and Pathogenesis
COVID-19 infection is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It belongs to the beta type of RNA coronavirus family. SARS-CoV-2 invades the host cell by the S spike proteins on the virus attaching to the angiotensin converting enzyme 2 (ACE2) receptor that is abundantly expressed on the epithelial cells within the lung, causing respiratory distress. The beta coronavirus S protein has also been known to interact with sialic acid membrane protein receptors on the host cell, which includes red blood cells (RBC). This suggests the virus can interact and interfere with the RBC membrane proteins integral to the structural integrity of the red blood cell. The structural membrane proteins provide flexibility that allows the blood cells to alter their shape to move through the narrow capillaries of the peripheral bloodstream to release oxygen to the body. SARS-CoV-2 causes structural membrane damage to the RBC, affecting their capacity to deliver oxygen. The sialic acid on the RBC membrane creates an overall negative electrical charge that causes the blood cells to repel each other and thus prevents the blood cells from attaching to one another. With the structural integrity of the membrane compromised, the red blood cells may exhibit abnormal morphological changes such as agglutination or rouleaux like presentation along with hemolysis.
Conclusion
The patient’s true hemoglobin level was reported to be 10.5 g/dL instead of the initial reading of 4.6 g/dL. A blood transfusion was no longer needed to treat the syncope and shortness of breath symptoms, thus the pRBCS were canceled. The physician narrowed down the differential diagnosis and the patient was treated for dehydration and sent home the same day once their vitals remained stable and constant. Although the laboratory discrepancies between the initial results and the final resultswere resolved using the advanced laboratory techniques, careful consideration should go into differential diagnosis in order to distinguish the results caused by COVID-19 from true pathological diseases such as cold agglutinin disease (CAD), cold autoimmune hemolytic anemia (CAIHA), paroxysmal nocturnal hemoglobinuria (PNH), paroxysmal cold hemoglobinuria (PCH), and other hematological and immunohematological diseases. Therefore, it is essential that not only the physician review the patient’s diagnosis and patient history, but the medical laboratory scientist does, too.
Summary
As more variants of COVID-19 continue to arise, more complications of COVID-19 continue to be discovered, even from the COVID-19 vaccinations. Careful consideration into how COVID-19 interferes with laboratory analysis should be examined as this affects pre- and post-analytical aspects such as turn-around times for physicians receiving patient results. Ultimately, this affects patient care and can delay treatment. This can cause the patient to be misdiagnosed or receive unnecessary blood products due to discrepant results caused by COVID-19.
References
- Mitra A., Dwyre D., Schuivo M., Thompson G.R., Cohen S., Ku N., Graff J.P. Leukoerythroblastic reaction in a patient with COVID-19 infection. American Journal of Hematology—Wiley Public Health Emergency Collection [Internet].2020 August. [cited 2020 April 6]; 95(8):999-1000. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7228283/ doi: 10.1002/ajh.25793
- Gupta R, Singh S, Anusim N, Gupta S, Gupta S, Huben M, Howard G, Jaiyesimi I. Coronavirus Disease 2019 and Cold Agglutinin Syndrome: An Interesting Case. European Journal of Case Reports in Internal Medicine [Internet]. 2021 March. [cited 2021 March 10]; 8(3):002387. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8112099/ doi: 10.12890/2021_002387
- Maslov DV, Simenson V, Jain S, Badari A. COVID-19 and Cold Agglutinin Hemolytic Anemia. Thrombosis and Hemostasis Open Access Journal [Internet]. 2020 July. [Cited 2020 August 20]; 4(3): e175-e177. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7440967/ doi: 10.1055/s-0040-1715791
- BayCare Health System, Department of Laboratory Services. i-STAT 1 Analyzer Procedure [Standard Operating Procedure]. i-STAT Corporation, New Jersey. Revised August 15, 2022.
- Thomas T, Stefanoni D, Dzieciatkowska M, Issaian Nemkov T, Hill R, Francis R, Hudson K, Buehler P, Zimring J, Hod E, Hansen K, Spitalnik S, D’Alessandro A. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. Journal of Proteome Research—American Chemical Socity Public Health Emergency Collection. [Internet]. 2020 November. [Cited 2020 October 26]; 19(11): 4455-4469. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7640979/ doi: 10.1021/as.jproteome.0c00606
- Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nature Review Immunology. [Internet]. 2020 April. [Cited 2020 April 26]; 20(6): 363-374. Available from: The trinity of COVID-19: immunity, inflammation and intervention – PMC (nih.gov) doi: 10.1038/s417577-020-0311-8
- Harmening D. The Antiglobulin Test. Modern blood Banking & Transfusion Practices. 7th F.A. Davis; 2019:103-118.
- Centers for Disease Control and Prevention. COVID-19 Treatments and Medications. [Internet]. [lasted updated 2022 August 5]. Available: COVID-19 Treatments and Medications | CDC
- U.S. Food & Drug Administration. Know Your Treatment Options for COVID-19. [Internet]. [lasted updated 2022 May 19]. Available: Know Your Treatment Options for COVID-19 | FDA
- Centers for Disease Control and Prevention. COVID-19 Testing: What You Need to Know. [Internet]. [lasted updated 2022 August 11]. Available: COVID-19 Testing: What You Need to Know | CDC
- U.S. Food & Drug Administration. COVID-19 Vaccines. [Internet]. [lasted updated 2022 August 19]. Available: Know Your Treatment Options for COVID-19 | FDA
- Douen A, George T, Ramsamooj K, Basit A, Kaur P, Shah T. A Cold Case of COVID-19-Induced Cold Agglutinin Disease. American College of Chest Physicians Journal. [Internet]. 2021 October. [Cited 2021 October 11]; 160(4): A880. Available from: A COLD CASE OF COVID-19-INDUCED COLD AGGLUTININ DISEASE – PMC (nih.gov) doi: 10.1016/j.chest.2021.07.823
- Harmening D. Detection and Identification of Antibodies. Modern blood Banking & Transfusion Practices. 7th ed. F.A. Davis; 2019:233-255.
- Hussain H, Sehring M, Singh Aulakh B. COVID-19-Associted Coagulopathy: A Case Report of Thrombosis Despite Therapeutic Anticoagulation. Case Reports in Critical Care. [Internet].2020 October. [Cited 2020 October 17];8876932. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7573660/ doi: 10.1155/2020/8876932
- Chowdhry E, Moshman J, Carroll S. A Case of COVID-19 Related Coagulopathy Complications and Heparin Resistance. The Cureus Journal of Medical Science. [Internet]. 2021 September. [Cited 2021 September 25]; 13(9): e18265. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8547601/ doi: 10.7759/cureus.18265
- Cui G, Li R, Zhao C, Wen Wang D. Case Report: COVID-19 Vaccination Associated Fulminant Myocarditis. Frontiers in Cardiovascular Medicine. [Internet]. 2021. [Cited 2022 Jan 24]; 8: 769616. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8818743/ doi: 10.3389/fcvm.2021.769616
- Zamzami O, Kabli AF, Alhothali A, Alhothali O, Alharbi T, Bahakim A, Marglani O. Post-COVID-19 Vaccine Parosmia: A Case Report. The Cureus Journal of Medical Science. [Internet]. 2021 December. [Cited 2021 December 9]; 13(12): e20292. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8747983/ doi: 10.7759/cureus.20292
- Ejigu T, Patel N, Sharma A, Vanjarapu JMR, Nookala V. Packed Red Blood Cell Transfusion as a Potential Treatment Option in COVID-19 Patients with Hypoxemic Respiratory Failure: A Case Report. The Cureus Journal of Medical Science. [Internet]. 2020 June. [Cited 2020 June 1]; 12(6): e8398. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7331927/ doi:10.7759/cureus.8398
- Harmening D. Adverse Effects of Blood Transfusion. Modern blood Banking & Transfusion Practices. 7th ed. F.A. Davis; 2019:373-395.
- Harmening D. Transfusion-Transmitted Diseases. Modern blood Banking & Transfusion Practices. 7th ed. F.A. Davis; 2019:307-332.
- BayCare Health System, Department of Laboratory Services. CBC with Automated Differential and Automated Reticulocyte Count (DXH) [Standard Operating Procedure]. Beckman Coulter, Inc., Fullerton, California. Revised March 22, 2022.
Jasmine Boykin is Clinical Laboratory Scientist for BayCare in Tampa, Florida.
 Case History
Case History