Volume 38 Number 4 | August 2024
Mahesh Percy, MBA, DMLT
Ronne Joseph, MD, MSc
Ewarld Marshall, MD, MScMED
Abstract
Myelodysplastic syndrome (MDS) is a group of blood cancers which interfere with the body’s ability to produce normal healthy blood cells. It was once thought to be a rare disorder, now recognized as the common hematologic neoplasm affecting more than 30,000 persons per year in the United States. This disease affects mostly the elderly population, and men are more commonly affected than women.1,15 The etiology of MDS is unknown. Here we present an 88-year-old woman with dysplasia of two cell (erythroid and myeloid) lineages. The patient is slightly anemic and has macrocytosis. This hematologic disorder is characterized by cytopenia and dysplasia in one or more cell lineage.2,12 The patient’s medical history revealed a gradual onset of fatigue, weakness, and increased susceptibility to infections. Diagnostic investigations, including peripheral blood smear, unveiled dysplastic changes across various cell lines, indicative of MDS. The diagnosis and classification are with the findings of dysplasia in one or more cell linage and blast cells in the bone marrow.3 It is essential to do bone marrow aspiration and cytogenetics to come to a final diagnosis. Given the patient’s advanced age, the patient refused to do bone marrow aspiration or cytogenetics. The primary purpose of this article on myelodysplastic syndrome (MDS) is to provide a comprehensive understanding of the disease, aiming to enhance diagnosis and knowledge.
Introduction
MDS is primarily a disease of the elderly, though it can affect individuals of any age. MDS constitutes a group of clonal disorders characterized by ineffective hematopoiesis, resulting in dysregulated production of blood cells. This introduction provides a foundational overview of MDS, setting the stage for a case study that explores the clinical manifestations associated with this condition.
MDS arises from genetic mutations and chromosomal abnormalities within the hematopoietic stem cells, leading to dysregulated differentiation and maturation of blood cells. This dysplasia manifests across various cell lines, including erythrocytes, granulocytes, and platelets. The severity of the disease can vary widely, and some cases may progress to Acute Myeloid Leukemia (AML).4
The clinical spectrum of MDS is diverse, ranging from asymptomatic cases discovered incidentally to severe manifestations characterized by fatigue, anemia, and a heightened susceptibility to infections. The diagnosis is established through a combination of clinical evaluation, peripheral blood smear, bone marrow examination, and genetic testing to identify specific mutations associated with MDS.5
The management of MDS encompasses supportive care, disease modifying therapies, and in some cases stem cell transplantation. However, the optimal treatment approach depends on factors such as disease subtype, risk classification, and patient comorbidities.6,16
According to the WHO classification in 2016 it recognizes six main types of MDS:
- MDS with multilineage dysplasia (MDS-MLD)
- MDS with single lineage dysplasia (MDS-SLD)
- MDS with ring sideroblasts (MDS-RS)
- MDS with excess blasts (MDS-EB)
- MDS with isolated del(5q)
- MDS unclassified (MDS-U)
Case Report
An 88-year-old Grenadian woman initially sought medical attention due to persistent fatigue and weakness, symptoms that had gradually developed over several months. She was a known diabetic and hypertensive patient. She also reported an increased susceptibility to infections, and these symptoms prompted a thorough investigation to understand the underlying cause of her deteriorating health. She has bilateral knee pain, no pulse appreciated in dorsalis pedis nor posterior tibial bilaterally. Complete blindness in the left eye due to a past surgical procedure 10 years ago. The patient also complains of bleeding gums after brushing. The patient had a history of well-managed hypertension and osteoarthritis, both of which were stable under the care of her primary care physician. There were no significant prior hematologic issues or malignancies documented in her medical history.
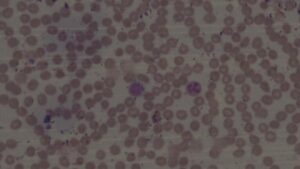
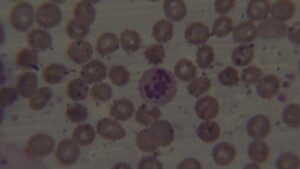
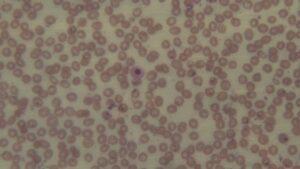
Peripheral smear report: The red cells (RBC) are decreased in number and are normochromic and macrocytic (oval-macrocytes and round macrocytes) with mild anisopoikilocyotsis. RBCs exhibit mild rouleaux formation. Occasionally a stomatocyte and rarely a tear drop cell was noted. Few target cells seen. Rarely a nucleated RBC and a polychromatic cell was noted. WBC series shows mild leukopenia. Some neutrophils are larger in size and show hypersegmented nuclei. Some neutrophils are dysplastic as evidenced by cytoplasmic hypogranularity and nuclear segmentation abnormalities. Platelets are adequate. No circulating blasts were seen.
The presence of dysplastic changes in both erythroid and myeloid linages, such as dysplastic neutrophils is particularly suggestive of Myelodysplastic Syndrome (MDS). However, it’s essential to note that these findings alone are not conclusive for a definitive diagnosis. Further diagnostic work-up, such as bone marrow aspiration and cytogenetic analysis, is crucial for confirming or ruling out myelodysplastic syndrome (MDS).
The patient refused to do bone marrow aspiration or cytogenetics.
Study Methods
When studying myelodysplastic syndrome (MDS), we employ various methods to investigate its epidemiology, pathophysiology, clinical manifestations, diagnostic approaches, treatment strategies, and prognostic factors. Here are some common study methods used in MDS.3
Epidemiological studies: Epidemiological studies aim to determine the incidence, prevalence, and risk factors associated with MDS. These studies often involve population-based cohorts, case-control studies, and retrospective analyses of medical records to identify demographic patterns, environmental exposures, and genetic predispositions linked to MDS development.
Pathological studies: Pathological studies involve the examination of bone marrow aspirates, biopsies, and peripheral blood smears to characterize morphological abnormalities, dysplastic changes, and cellular dysfunctions associated with MDS. Histological analyses, flow cytometry, immunohistochemistry, and cytogenetic studies help in MDS diagnosis, subtype classification, and risk stratification.
Prognostic studies: Prognostic studies aim to identify clinical, genetic, and molecular factors predictive of disease progression, treatment response, and overall survival outcomes in MDS patients.
Discussion
The case study of an MDS diagnosis in an 88-year-old woman introduces unique considerations related to age, comorbidities, and the overall health status of the patient. This section will investigate specific aspects relevant to an elderly patient with MDS including diagnostic challenges, treatment decisions, and the impact on patient’s quality of life. In this case the ineffective hematopoiesis leading to cytopenia and dysplasia in more than one cell lineage and there are increased chances of progression to AML.7,13
Prognosis in MDS varies widely, influenced by factors such as age, overall health, specific subtype of MDS, and the severity of cytopenias. Given the advanced age, the impact of comorbidities on prognosis becomes significant. Treatment decisions in elderly patients need to consider their overall health and ability to tolerate therapies. Options may include supportive care (blood transfusions, growth factors), hypomethylating agents, or, in some cases, stem cell transplantation.8,9,14 Discussions should encompass the patient’s goals, preferences, and the potential impact of treatment on her quality of life.
The balance between managing the disease and maintaining comfort is crucial, especially in elderly individuals. Regular monitoring of blood counts and disease progression is essential.
Treatment strategies may need to be adjusted based on the patient’s response and any side effects experienced. Considering the emotional and psychological aspects of the diagnosis is vital. Support from family, friends, and potentially counseling services can play a crucial role in helping the patient cope with the challenges associated with MDS.10 It’s important for the healthcare team, including hematologists and geriatric specialists, to work collaboratively to provide holistic care tailored to the individual needs and circumstances of the 88-year-old woman with MDS.11
Conclusion
From this study, the morphological findings are helpful to reach the diagnosis, but bone marrow aspiration would give a definitive diagnosis. Because of the patient’s unwillingness to do further investigations we couldn’t come to a final conclusion.
The study of myelodysplastic syndrome (MDS) in an 88-year-old woman underscores the complexity of managing this hematologic disorder in the elderly population. The comprehensive diagnostic approach, considering blood tests, bone marrow biopsy, and cytogenetic analysis, aids in understanding the disease’s subtype, contributing to prognosis assessment.
Given the advanced age of the patient, the clinical presentation is characterized by symptoms related to cytopenias, necessitating a careful balance between disease management and maintaining quality of life. Prognosis, influenced by factors like age, overall health, and subtype severity, underscores the need for personalized treatment decisions.
In conclusion, managing MDS in an elderly individual requires a multidisciplinary approach that prioritizes individualized care, considering not only the disease characteristics but also the patient’s unique circumstances, goals, and preferences. This study contributes to the broader understanding of MDS in the elderly and emphasizes the importance of tailoring interventions to optimize both medical outcomes and overall quality of life.
References
- Alessandrino EP, Amadori S, Cazzola M. Myelodysplastic syndromes: recent advances. Haematologica. 2001 Nov;86(11):1124-57.
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872–85.
- Germing U, Gattermann, Strupp C. Validation of the WHO proposals for a new classification of primary myelodysplastic syndromes: a retrospective analysis of 1600 patients. Leukemia Research. 2000;24:983-992.
- Rollison DE, Howlader N, Smith MT. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008 Jul 1;112(1):45-52.
- Hellström-Lindberg E, Willman C, Barrett J, Saunthararajah Y. Achievements in understanding and treatment of myelodysplastic syndromes. American Society of Hematology Education Program Book. 2000:110-132.
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088.
- Bernasconi P, Klersy C, Boni M, et al. World Health Organization classification in combination with cytogenetic markers improves the prognostic stratification of patients with de novo primary myelodysplastic syndromes. Br J Haematol. 2007 May;137(3):193-205.
- Niscola P, Palombi M, Trawinska M, Tendas A, Giovannini M, Scaramucci L, Perrotti A, Fabritiis P. Managing myelodysplastic syndromes in very old patients: a teaching case report. Clinical Interventions in Aging. (2013); 8: 391-394.
- Niscola P, Palombi M, Trawinska M, Tendas A, Giovannini M, Scaramucci L, Perrotti A, Fabritiis P. Managing myelodysplastic syndromes in very old patients: a teaching case report. Clinical Interventions in Aging. (2013); 8: 391-394.
- Cheson BD. The myelodysplastic syndromes: current approaches to therapy. Ann Intern Med. 1990 Jun 15;112(12):932-41.
- Ria R, Moschetta M, Reale A, Mangialardi G, Castrovilli A, Vacca A, Dammacco F. Managing myelodysplastic symptoms in eldery patients. Clinical Interventions in Aging. (2009); 4: 413-423.
- Ma X, Does M, Raza A, Mayne S. Myelodysplastic syndromes. Cancer. (2007); 109(8): 1536-1542.
- Stauder R. The challenge of individualized risk assessment and therapy planning in eldery high-risk myelodysplastic syndromes (MDS) patients. Annals of Hematology. (2012); 91(9): 1333-1343.
- Schumacher HR, Nand S. Myelodysplastic Syndromes: Approach to Diagnosis and Treatment. New York: Igaku-Shoin Medical Publishers; 1995
- Cazzola M, Malcovati L. Myelodysplastic syndromes—coping with ineffective hematopoiesis. N Engl J Med.2005; 352: 536–538.
- Bennett J. The myelodysplastic syndromes: pathobiology and clinical management. In: B Cheson, editor Basic and Clinical Oncology, vol. 27 New York: Marcel Dekker; 2002: 3–7.
Mahesh Percy is an Instructor/Medical Technologist at St. George’s University in St. George’s, Grenada.
Dr. Ronne Joseph is a Clinical Instructor at St. George’s University in St. George’s, Grenada.
Dr. Ewarld Marshall is Chair, Pathology at the Medical Pathology Diagnostic Lab Director at St. George’s University in St. George’s, Grenada.
This article was previously published by Acta Scientific Clinical Case Reports in Volume 5 Issue 5 May 2024.
Lab Reports
- Hemoglobin – 11.8 g/dl* (12–16 g/dl)
- RBC count – 2.68 10*6/ul* (4.0–5.5 10*6/ul)
- Hematocrit – 34.5%* (37–47%)
- MCV – 128.7 fL* (82–98 fL)
- MCH – 44.1 pg* (26–34 pg)
- MCHC – 34.3g/dl (31–38 g/dl)
- RDW – 12.1% (11.6–13.7%)
- MPV – 10.5fL (7.8 – 11.0 fL)
- WBC count – 4.4 10*3/ul* (4.5–11.0 10*3/ul)
- Neutrophils – 58.4% (40–70%)
- Lymphocytes – 33.7% (20–40%)
- Eosinophils – 1.1% (1–4%)
- Monocytes – 6.3% (2–8%)
- Basophils – 0.5% (0–1%)
- ESR – 58 mm/hr* (0–20 mm/hr)
- Glucose (FBS) – 191.2 mg/dl* (60–110 mg/dl)
- BUN – 19.4 mg/dl* (7–18 mg/dl)
- Creatinine – 0.83 mg/dl (0.4–1.4 mg/dl)
- Uric acid – 3.5 mg/dl (2.5–7.7 mg/dl)
- Total Bilirubin – 0.31 mg/dl (0.2–1.2 mg/dl)
- Direct Bilirubin – 0.16 mg/dl (0.0–0.30 mg/dl)
- AST(SGOT) – 21.2 IU/L (5–34 IU/L)
- ALT(SGPT) – 12.1 IU/L (4–36 IU/L)
- Alkaline phosphatase – 75.9 IU/L (35–123 IU/L)
- GGT – 19.1 U/L (9–39 U/L)
- Total Protein – 7.7 g/dl (6.6–8.8 g/dl)
- Albumin – 4.3 g/dl (3.5–5.2 g/dl)
- Total Cholesterol – 184 mg/dl (135–200 mg/dl)
- HDL – 43.8 mg/dl (>40 mg/dl)
- Triglycerides – 115 (40–140 mg/dl)
- CHOL/HDL ratio – 4.20 ratio (<5.60 ratio)
- LDL – 117 mg/dl (<130 mg/dl)
- VLDL – 23 mg/dl (0–40 mg/dl)
- HbA1c – 6.0% (<6.0%)
- Ferritin – 94.1 ng/ml (5–148 ng/ml)
- Vitamin B12 – 809 pg/ml (174–878 ug/ml)
- Iron – 95.0 ug/dl (37–145 ug/dl)
- TIBC – 332.0 ug/dl (250–450 ug/dl)
- % Saturation – 28.6% (20–55%)